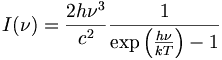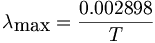Radiant Heating Formulas
11/12/13
|
Planck's Law Stephan-Boltzman Law Wien's Law Kirchhoff's Law
|
|
Wien's Law Wave length is inversely proportional to temperature of the source. The relationship of the wavelength of maximum intensity of a black body to its absolute temperature is expressed by Wien's law. Wien's Law
L max = a/T a = 2898 if L is measured in microns
|
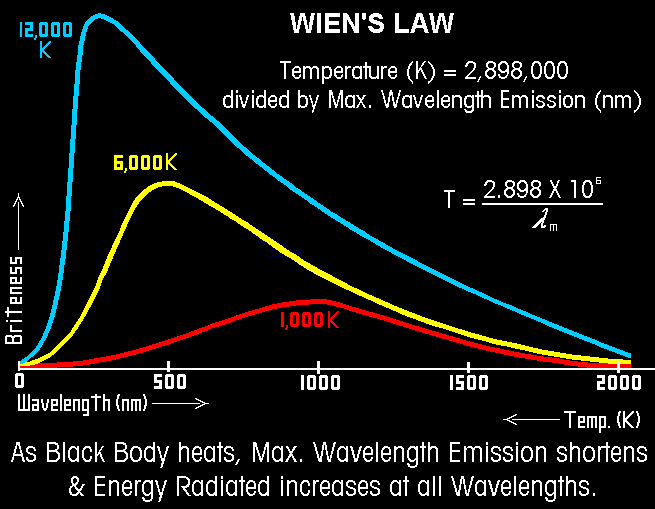 From the Amherst Astronomy Assoc. with thanks to Mark Godwin at GSSM
|
|
Kirchhoff's Law
This can be expanded into the "common sense" equation that the total incident energy must equal the sum of the absorbed, reflected, and transmitted energy.
2) Be reflected (the opposite of a perfect black body - a material with low emissivity) 3) Pass through the material (transparancy)
|
|
Absolute Zero The lowest possible temperature allowed by the laws of thermodynamics. At this temperature, molecules would possess the absolute minimum kinetic energy allowed by quantum mechanics (the Heisenberg Uncertainty Principle places a greater than zero lower limit on the kinetic energy of molecules). It is equivalent to -273.15 deg.C or 0K (kelvin). At absolute zero, the entropy of any system vanished.
Absolute Zero in K=C+273.16 |

Radiant Heat
Buckley Rumford Fireplaces
Copyright 1995 - 2013 Jim Buckley
All rights reserved.
webmaster